Corrosion Protection for Fastenings
Information about effective corrosion protection for timber fastenings
CORROSION PROTECTION FOR FASTENINGS
The purpose of a fastener: To securely fix one building element to another.
Examples of timber fasteners: Nails, screws, nail plates
To be effective: The fastener must maintain its full tensile strength which keeps the two building elements under tight contact pressure.
Failure: The contact pressure and resulting frictional forces between building elements is lost. If the fastening completely fails the joint has no value to the structure whatsoever.
Corrosion is a leading cause of fastening Failure: Fastening corrosion is usually the reaction of steel with oxygen to form iron oxide, which is rust.
The Fasteners themselves: Nails, nail-plates and screws are often made from steel. Steel is one of the strongest fastening materials, but in some environments steel can be highly prone to corrosion. If corrosion is permitted to continue the metal will weaken and fail.

Some metals will protect steel from corrosion. This is done in two ways:
1.Barrier method:Some metals form an oxide layer on the surface that will not dislodge. The barrier will prevent oxygen from reaching base metal underneath – no oxygen, no corrosion.
Both Chromium and Zinc act as a barrier to prevent oxygen reaching steel.
Galvanising: Zinc can be applied over steel either by the hot dip method or by electro-galvanising to coat the steel and protect it from the corrosive elements. Hot dip galvanising provides a much thicker and longer lasting protective layer than does the electro-galvanising method.
Stainless steel: Stainless Steel is an alloy of chromium and behaves quite differently to galvanising. Above 13% the chromium will be sufficient to oxidise on the surface to form an impenetrable barrier preventing oxygen reaching the steel.
Stainless steel can corrode if there is insufficient oxygen to allow the chromium to form a surface barrier and chlorides (such as salt water) are present. This is called crevice corrosion.
2. Sacrificial (Cathodic) Protection: This is where one metal will protect another by corroding first. The technical term for this is cathodic protection. On the following chart, any metal toward the left that is in contact with another metal to its right, will “sacrifice” itself in order to protect the metal to its right. Zinc is to the left of steel so protects the steel.
Sacrificial (Cathodic) Protection of Metals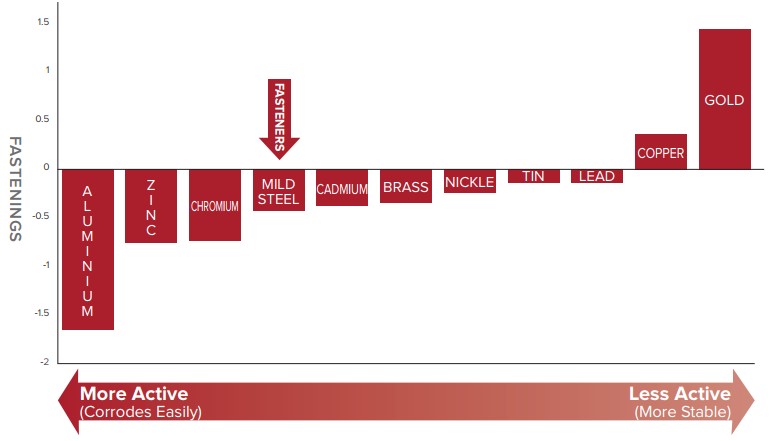
How does this apply to wood?
Corrosion occurs fastest where a catalyst is present. A catalyst can be a mixture of moisture, steam, volcanic activity, acids, or salt.
Acids: Depending on the species, wood contains natural acids. The acidity of Pinus Radiata is less severe in this respect than many, but some species have levels high enough to cause concern if moisture is present as high moisture levels can activate the acid.
Volcanic Activity: In geothermal areas the requirement is often for stainless steel fasteners to provide durability against the sulphur. This is also why CCA timber in a volcanic area turns black – the copper in the treatment reacts with the sulphur. Steel fasteners will also suffer if not protected.
Salt: Salt is a particularly active catalyst and is carried by spray near coastal New Zealand. This is why stainless steel fasteners are a requirement in these areas.
Preservation Chemicals: Boron does not pose a risk for fasteners when in a dry internal frame with MC< 20%. Bright steel nails have successfully been used for decades in this environment. CCA H3.2 timber is similar in that bright steel nails will not corrode provided a low moisture content is maintained. However, it must be assumed that the higher level of treatment (CCA H3.2 or higher) has been chosen with anticipation of becoming a wet area or being exposed to external moisture. Treatments H4 or higher contain much higher levels of copper which will encourage mild steel to corrode faster.
Galvanised or stainless steel fasteners should be used wherever CCA timber is specified, regardless of whether the application is internal or external, wet or dry.
Bright steel screws and nails should be used only in areas where the timber can be guaranteed to remain perfectly dry throughout its service life and not exposed to any of the catalysts listed above. In all other areas galvanised or stainless fasteners should be used and the requirements of NZS3604 followed closely.
